Features & Benefits:
Built-in system and database redundancy for compliance and peace of mind:
– Particle counter data buffer redundancy for unexpected communications failure
– Secure, high availability, high speed SQL databases enables real-time mirror database back up
– Buddy option, complete system automatic failover functionality on computer failure, no manual intervention required.
Meets all regulatory guidelines for GMP lifescience applications:
– Fully GAMP® compliant.
– Enables 21 CFR Part 11 compliance.
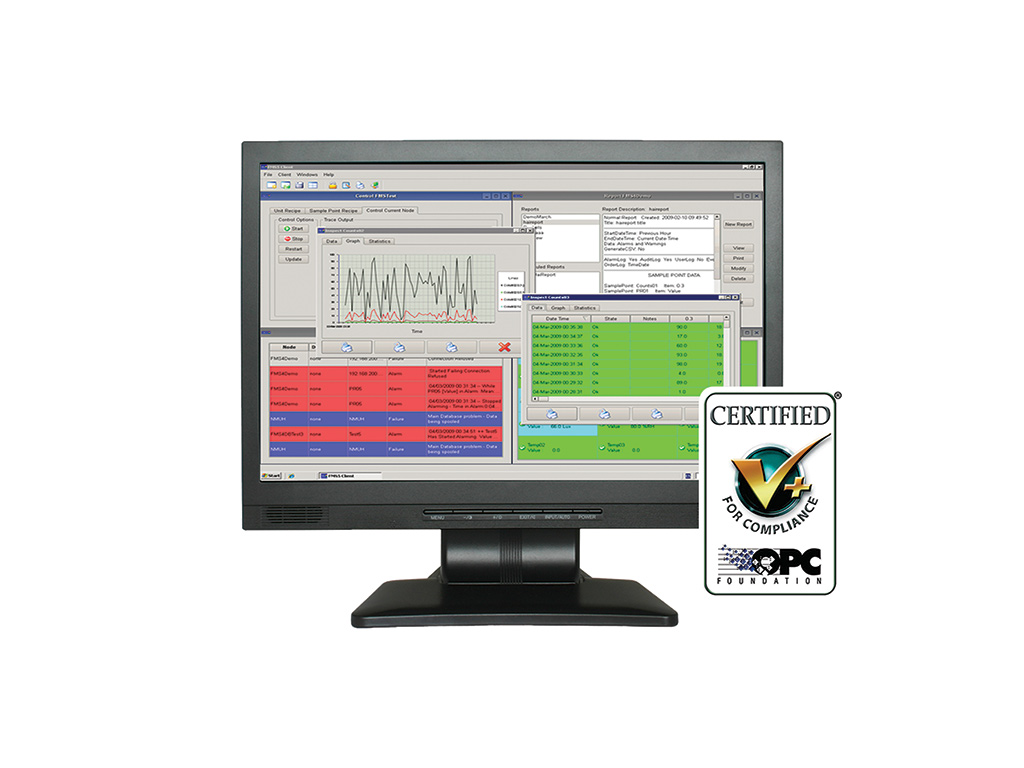
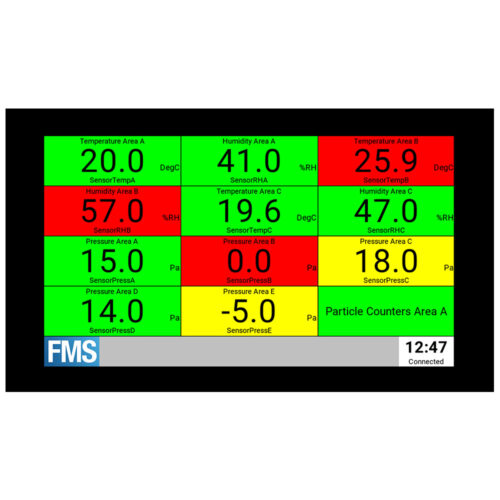
Intuitive operation ensures the right information reaches the right people:
– Alarm display, notification (email. SMS and telephone) and acknowledgement.
– User configurable system views, status windows and multi-level maps.
Demonstrates compliance using powerful user defined reporting, auto reporting and easy data export tools.
Technical Specifications:
Any device that has a linear analog output can be integrated into FMS including:
AeroTrak Remote Particle Counters.
AeroTrak Portable Particle Counters.
AeroTrak Cleanroom Condensation Particle Counter.
BioTrak Real-Time Viable Particle Counter.